On this page, We are going to learn about the full form of pH and the meaning of pH, As well as the meaning, definition, abbreviation, and acronym for pH in different categories. So you should read this post till the end.
The Full Form of pH – Potential of Hydrogen
pH Stands for Potential Hydrogen. The pH scale measures the amount of acidity or alkalinity in a solution. The pH scale measures the amount of hydrogen (positive) or hydroxyl (negative) ions.
The pH scale ranges from 0 to 14, A pH of 7 is neutral, while a pH of 14 means the property of the solution is very acidic and a pH of 1 means the property of the solution is very alkaline.
A lower pH indicates a more acidic solution and a higher pH indicates a more alkaline solution. The pH of a solution can be adjusted by adding or removing a base or acid.
| Formula | pH=-log10[H+] |
| Discovered | 1909 |
| Discovered By | Sren Peder Lauritz Srensen |
| PH Value Range | 0 to14 |
| Types of solutions | The neutral solution, Alkaline solution, Acidic solution |
What is the PH value in chemistry?
The pH value shows the concentration of hydrogen ions present in any one solution. PH value is used to find out the nature of the solution that which is acidity and alkalinity.
the pH value calculated on the PH scale. If the pHs of less than 7 indicates acidity in nature. but if the pH value of a solution is 7 then it is neutral in nature. if the pH value of a solution is more than 7 then it is alkaline in nature.
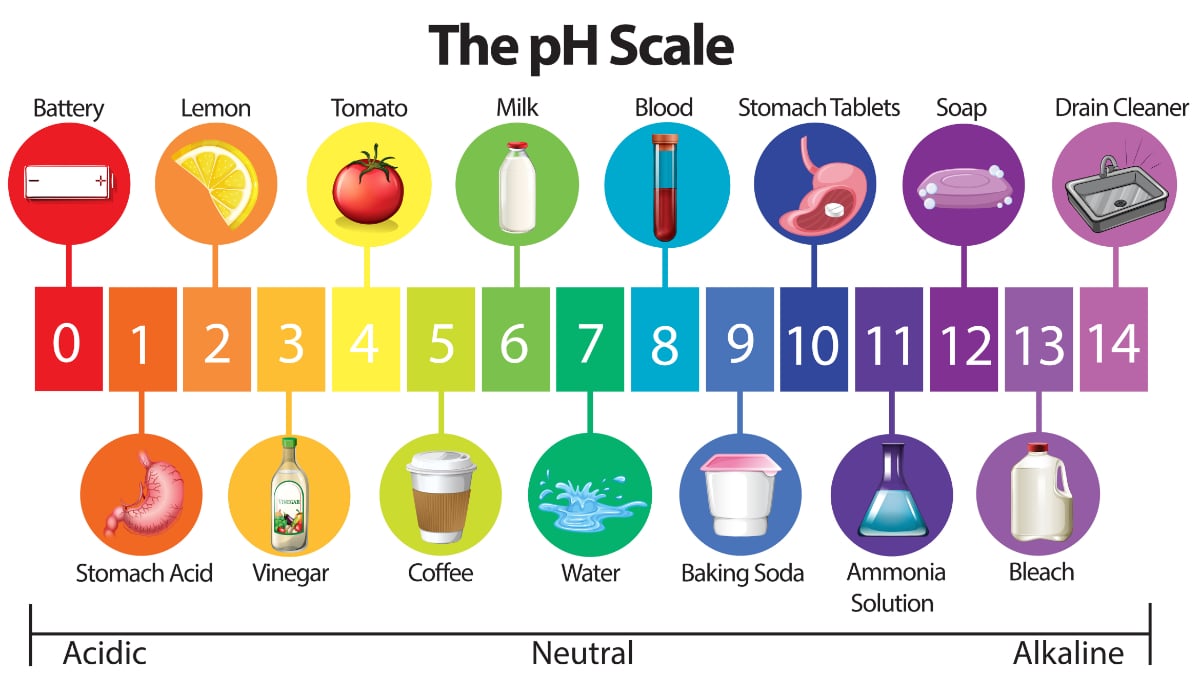
What is the ph scale in chemistry
the ph scale is a scientific instrument that is used to measure the acidity, basicity, or neutrality of a solution. It is measured as the negative logarithm of the number of hydrogen ions present in a solution.
The PH scale was discovered in 1909 by Sren Peder Lauritz Sorensen, a Danish chemist at Carlsberg Laboratory, who is known as the inventor of the PH scale.
- the scale range of the pH scale is between 0 to 14.
- PH scale range 0 indicates highly acidic
- PH scale range 14 indicates highly alkaline
- pH scale range 7 indicates neutral
- pH is less than 7 are acidic.
- pH greater than 7 indicates alkaline.
Why pH is not more than 14?
pH is not more than 14? pH is a measure of the concentration of hydrogen ions in a solution. pH is a scale from 0 to 14, with 7 being neutral.
A solution with a pH of 7 is neutral, meaning there are equal numbers of hydrogen (H+) and hydroxide (OH-) ions in the solution. let’s understand, Why the pH is not more than 14?
we measure the pH of all of the samples in water. And the reaction of water is H20 = (H+) + (OH-)
- In this equilibration constant kw is
- [H+] [OH-] = Kw = 1 X 10^-14
- Now, By applying -log on both sides
- -log [OH-] – log [H+] = 14
- According to the formula pH= – log [H+]
- Therefore, pH = 14 – (-log [OH-])
- Well, this equation proves that the higher the concentration of OH- ions, the lower the value of -log [OH-].
- If the concentration of OH- ions reaches 1 mol/dm3, the value of -log [OH-] is zero, which, according to the equation, gives pH=14.0.
- However, if the concentration of OH- ions exceeds 1 mol/dm3 (which is very rare), the value of -log [OH-] would be negative,
- which, according to the equation, gives a pH of greater than 14.0.
List of important Ph value chart
there are some examples of ph value chart, that helps to find out, of property of daily uses of food and things.
| Substance | Ph Value |
|---|---|
| Hydrochloric acid (HCl) | 0 (most acidic) |
| Stomach acid | 1 |
| Lemon | 1.8 – 2 |
| Lemon juice | 2 |
| Cola, beer, vinegar | 3 |
| Tomatoes | 4 |
| Coffee | 5 |
| Normal rainwater | 5.5 |
| Urine (human urine) | 6 |
| Saliva (human saliva) | 6.5 |
| Pure Water | 7 (indifferent) |
| Human blood | 7.5 |
| Seawater | 8 |
| washing soda (Na2CO3 ) | 9 |
| Great Salt Lake | 10 |
| Ammonia | 11 |
| Sodium Bicarbonate (Baking Soda – NaHCO₃) | 12 |
| Oven cleaner | 13 |
| Sodium hydroxide (caustic soda- NaOH ) | 14 (most alkaline) |
| Milk | 6.5 – 6.7 |
| Acid rain | 2 to 5.6 |
| Wines | 2.8 to 3.8 |
| Black Coffee | 5 |
| Butter | 6.1- 6.4 |
| Tea | 5.5 |
| Salt ( common salt -sodium chloride ) | 7 |
FAQs and the meaning of pH?
-
What is the pH Full form in chemistry
pH is known as the potential of hydrogen. The first time theory of pH was presented by Sren Peder Lauritz Sorensen in 1909. The Definition of pH and all possible meanings are given above, You might also like some similar terms related to pH.
-
What is the pH formula?
The formula to calculate pH is: pH = -log[H+]
-
Is pH 7 an acid or base
The pH ranges from 0 to 14, a pH value of 7 indicates neutral. pH less than 7 is acidic while pH greater than 7 is alkaline.
-
What is the ph value of Pure water?
PH value of water, there can be two types of water in it.
• Normal Water (Ph Value Of Normal Drinking Water) = 6.5 – 8.5
• Pure water (Ph Value of Pure Water) = 7 at 25 °C
• Rain Water (Ph Value Of Rain Water) = 5.0 – 5.5
• Acid rainwater (Ph Value Of Acid Rain Water) = 4.0. -
What is the Ph Value of Human Blood
The Ph range of human blood is 7.35 to 7.45, which means the blood is slightly alkaline. Many types of health problems can occur due to the increase or decrease of pH in the blood, when the pH value of a person’s blood becomes 0.2, then he dies.
If the pH of the blood is below 7.35 then the blood is acidic and the blood pH is more than 7.45 and becomes too alkaline, balancing this imbalance in the blood to the lungs and kidneys.
The lungs remove carbon dioxide from the human body through respiration and the kidneys remove the acid from the body through urine or excretion. In this way, both these organs balance the pH value of human blood.
-
What is the pH value of lemon?
Often the taste of lemon is sour, or rather it is acidic in nature. Therefore it is natural that the PH value of a lemon will be less than 7.
• The pH value of lemon is 1.8 – 2.
• The pH value of lemon juice is 2.00–2.60.
• PH value of Lemon = 1.8 – 2
• PH value of Lemon juice = 2.00–2.60
• PH value of Orange, pH 4.35 -
What is the pH value of Milk?
Milk is used in making many types of milk products such as cheese, ice cream, butter, and curd, but before using it,
the PH value of milk should be known. Because due to a change in the pH value of milk, it gets spoiled.
Ph Value Of Milk = 6.7 to 6.9
Almond Milk PH Levels = 6 -
What is the pH value of human urine
PH value of Urine is 6. A urine test is done to detect many types of diseases in the human body. Since urine contains water, salt, and waste products, all of these compounds can affect acidic levels.
With the help of this test, experts measure pH, and also find out the pH value of human urine – pH value of human urine – is 6. It can range from 4.5 to 8.0. Urine less than 5.0 is acidic, and urine greater than 8.0 is alkaline
-
What is the pH value of Saliva
Normal PH Range For Saliva = 6.2 To 7.6
Saliva plays a very important role in our digestion process, by chewing food in the mouth, saliva mixes with it and from there the digestion of food starts,the normal pH range of human saliva = 6.2 to 7.6
An imbalance in the pH value of saliva can cause health issues like disturbances indigestion, tooth cavities, bad breath, and sensitivity to hot or cold in the teeth.
